Background
Improvements in disease management techniques have led to increases in the life expectancy of individuals with sickle cell disease (SCD). Despite the increasing number of older adults with SCD (defined as age ≥ 40 years), there is limited data on how symptoms experienced by this population such as pain, sleep, depression, and fatigue impact how they function day-to-day. In this study, we sought to determine which modifiable patient-reported factors most strongly correlate with patient reported physical function in adults with SCD. Understanding factors associated with impaired physical function will provide targets for future interventional studies to improve function.
Methods
We conducted a cross-sectional study at a single academic sickle cell center. Participants completed the Adult Sickle Cell Quality of Life Measurement Information System (ASCQ-Me) Stiffness Impact and Sleep Impact surveys and the Patient-Reported Outcomes Measurement Information System (PROMIS) Physical Function, Pain Intensity, Pain Interference, Fatigue, Cognitive Function, and Emotional Distress measures. For ASCQ-Me, higher scores imply better self-reported health. For PROMIS, higher scores mean higher levels of the concept being measured. Relationships between the variables thought to influence SCD experience were explored using analytical networks and centrality measures (INSIGHTS, Nanbar Health's data analysis and 3D visualization tool) that built upon calculated pairwise Spearman correlations of the non-normal variable set.
Results
Surveys were administered to 92 participants with 97% of participants completing all surveys. The mean score for each item was obtained across all participants. Scores were then stratified by age group and t-tests were performed to compare scores on results between groups for each ASCQ-Me and PROMIS measure. Across those who completed both surveys, the mean age was 43 (SD 13.7). The mean age for older adults was 54 (SD 8.6) and the mean age for younger adults was 30 (SD 5.5). More participants were female (58%) than male (42%). Genotypes included HbSS (58%), HbSβ 0-thalassemia (5%), HbSC, (24%), and HbSβ +-thalassemia (13%).
The mean score on PROMIS Physical Function for all participants was 48.7 with 18% of responders reporting impaired physical function (score ≤ 40). Older adults had lower mean scores on PROMIS Physical Function (46.4) vs. for younger adults (51.6, p <0.05).
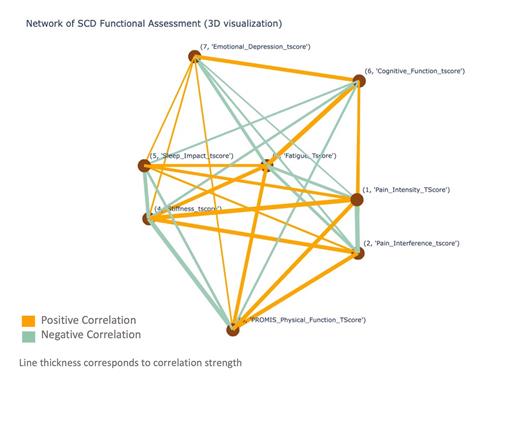
The strongest correlations were between PROMIS Physical Function and ASQME Stiffness Impact (r s= 0.66; p<0.001), PROMIS Pain Intensity (r s= -0.61; p<0.001), PROMIS Pain Interference, (r s= -0.61; p<0.001), and ASCQ-Me Fatigue (r s = -0.55; p<0.001). There was a moderate positive correlation between PROMIS Physical Function and ASCQ-Me Sleep Impact (r s= 0.40; p<0.001) and PROMIS Cognitive Function (r s=0.36 p < 0.001) implying better sleep and better cognitive function were associated with better patient reported physical function. Lastly, better physical function had a low-moderate association with PROMIS Emotional Distress (r s= - 0.24; p < 0.05).
We assessed relationships between all of the patient-reported outcome measures and found that the strongest relationships were between Pain Intensity and Pain Interference (r s= 0.69), Pain Intensity and Stiffness (r s= -0.67), and Fatigue and Cognitive Function (r s= -0.69). There was no central node identified (Figure).
Conclusion
Each factor evaluated in this study showed a statistically significant and clinically meaningful relationship to patient reported physical function. One of our major findings was that when mapped against each other, there was no central node explaining the identified correlations between the variables assessed. These results suggest that physical functioning is multifactorial and that there exist multiple modifiable factors that may influence function in people with SCD. Factors associated with physical function will serve as targets for future interventional studies to maintain and improve function in adults with SCD.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal